您好!歡迎訪問洛陽富道生物科技有限公司官方網(wǎng)站!

Current clinical applications of mesenchymal stem cell therapy for osteoarthritis lack consistency because there are no established criteria for clinical processes. We aimed to systematically organize stem cell treatment methods by reviewing the literature. The treatment methods used in 27 clinical trials were examined and reviewed. The clinical processes were separated into seven categories: cell donor, cell source, cell preparation, delivery methods, lesion preparation, concomitant procedures, and evaluation. Stem cell donors were sub-classified as autologous and allogeneic, and stem cell sources included bone marrow, adipose tissue, peripheral blood, synovium, placenta, and umbilical cord. Mesenchymal stem cells can be prepared by the expansion or isolation process and attached directly to cartilage defects using matrices or injected into joints under arthroscopic observation. The lesion preparation category can be divided into three subcategories: chondroplasty, microfracture, and subchondral drilling. The concomitant procedure category describes adjuvant surgery, such as high tibial osteotomy. Classification codes were assigned for each subcategory to provide a useful and convenient method for organizing documents associated with stem cell treatment. This classification system will help researchers choose more unified treatment methods, which will facilitate the efficient comparison and verification of future clinical outcomes of stem cell therapy for osteoarthritis.

The purpose of this study was to increase the consistency of future MSC therapies for OA by categorizing the current treatment methods.
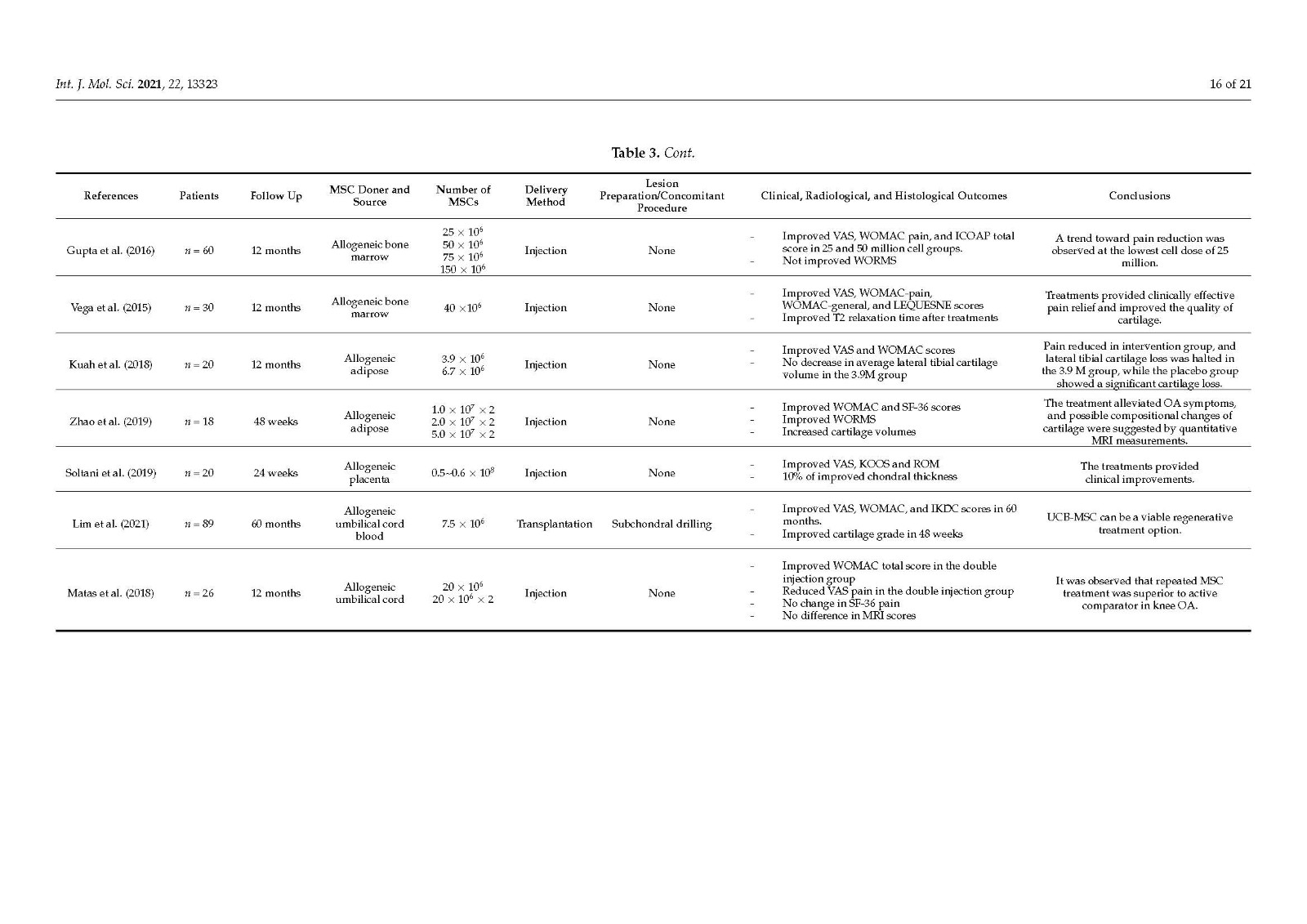
The treatment methods can be divided into seven categories: cell donor, cell source, cell preparation, delivery methods, lesion preparation, concomitant procedures, and evaluation. Based on these procedures, classification codes were assigned to each subcategory. Stem cell donors were subdivided into autologous and allogeneic, and stem cell sources included bone marrow, adipose tissue, peripheral blood, synovium, placenta, and umbilical cord. MSCs were prepared through cell expansion or isolation processes. They were attached to cartilage defects using matrices or injected into the joints under arthroscopic observation. The lesion preparation category was divided into three subcategories: chondroplasty, microfracture, and subchondral drilling. The concomitant procedure category describes adjuvant surgery, such as high tibial osteotomy.
Additional parameters can be added in future clinical studies. Cell sources can include MSCs from molar cells, amniotic fluid, and induced pluripotent stem cells. Some alternative cell sources, such articular cartilage progenitors and chondrogenic progenitor cells that have the potential to treat OA can also be considered as new parameters [74,75]. The addition of various treatment factors, such as PRP, hyaluronic acid, and some growth factors, can be considered as a new category. Low-intensity pulsed ultrasound (LIPUS), meniscectomy, and anterior cruciate ligament (ACL) reconstruction can be attempted as additional concomitant procedures. Since current MSC therapies are inconsistent and lack homogeneity, the classification system proposed in this study is expected to facilitate the efficient comparison and verification of clinical outcomes from MSC therapy for degenerative OA. Furthermore, if the analysis of the clinical results for each category is accumulated, the optimal combinations of efficient stem cell treatment methods can be found.
本研究的目的是通過對當(dāng)前治療方法進(jìn)行分類,提高未來 MSC 治療 OA 的一致性。
治療方法可分為七類:細(xì)胞供體、細(xì)胞來源、細(xì)胞制備、遞送方法、病變制備、伴隨程序和評估。根據(jù)這些程序,為每個子類別分配了分類代碼。干細(xì)胞捐獻(xiàn)者分為自體和異體,干細(xì)胞來源包括骨髓、脂肪組織、外周血、滑膜、胎盤和臍帶。MSCs 是通過細(xì)胞擴(kuò)增或分離過程制備的。它們使用基質(zhì)附著在軟骨缺損處或在關(guān)節(jié)鏡觀察下注射到關(guān)節(jié)中。病變準(zhǔn)備類別分為三個子類別:軟骨成形術(shù)、微骨折和軟骨下鉆孔。伴隨手術(shù)類別描述了輔助手術(shù),例如脛骨高位截骨術(shù)。
可以在未來的臨床研究中添加其他參數(shù)。細(xì)胞來源可以包括來自磨牙細(xì)胞、羊水和誘導(dǎo)多能干細(xì)胞的 MSC。一些替代細(xì)胞來源,例如具有治療 OA 潛力的關(guān)節(jié)軟骨祖細(xì)胞和軟骨祖細(xì)胞,也可被視為新參數(shù) [ 74 , 75]]。各種治療因子的加入,如PRP、透明質(zhì)酸,以及一些生長因子,可以算是一個新的品類。可以嘗試低強(qiáng)度脈沖超聲 (LIPUS)、半月板切除術(shù)和前交叉韌帶 (ACL) 重建作為額外的伴隨手術(shù)。由于目前的 MSC 療法不一致且缺乏同質(zhì)性,因此本研究中提出的分類系統(tǒng)有望促進(jìn) MSC 治療退行性 OA 的臨床結(jié)果的有效比較和驗證。此外,如果累積對每個類別的臨床結(jié)果的分析,可以找到有效干細(xì)胞治療方法的最佳組合。
關(guān)鍵詞: 退行性骨關(guān)節(jié)炎,干細(xì)胞療法,膝蓋,軟骨再生,degenerative osteoarthritis,stem cell therapy, knee, cartilage regeneration
來 源: MDPI https://www.mdpi.com/1422-0067/22/24/13323/htm











上一篇: 細(xì)胞工廠內(nèi)支原體污染的四種檢測方法
下一篇: 血清瓶有哪些特點(diǎn)